A new RP-HPLC method for metformin determination in human serum
Main Article Content
Abstract
Background: Anticoagulant in the sample bottles could form complex with metformin thereby affecting its concentration. This necessitates the need to develop HPLC method for determination of metformin in serum
Objectives: This study was aimed at developing a simple, less tedious RP-HPLC method for the determination of metformin in human serum.
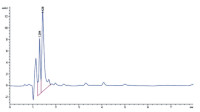
Methods: Blank serum (2 mL) was spiked into solution containing 2 mL metformin (2-65 µg mL-1) and 1 mL caffeine (5.0 µg mL-1) as internal standard (IS) centrifuged at 3000 rpm for 5 minutes. Portion (0.5 mL) of the resultant solution was injected into the HPLC auto sample machine (Agilent 1260 infinity). The optimized conditions included a mobile phase of methanol-water (80:20 v/v) containing 0.1 % orthophosphoric acid, isocratic elusion mode, an injection volume of 10 µL, flow rate of 1.2 mL min-1, at 35 °C and detection wavelength of 255 nm. Calibration curve (0.40 to 12.2 µg mL-1) was constructed by plotting the peak area ratios against their corresponding concentrations. The method was validated according to ICH guidelines.
Results: Metformin and caffeine eluted at 1.29 and 1.42 minutes respectively. The method was precise (4.77 % RSD), accurate (% Er of 0.22 and % recovery of 99.78 %) with a linear calibration curve (r = 0.988). LOD and LOQ of the developed method are 1.93 and 5.87 µg/mL respectively. All the parameters were within the acceptable limits.
Conclusion: The developed method was found to be simple, precise, accurate and rapid for the routine therapeutic metformin monitoring in human serum.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
Sweetman SC (2002). Martindale. The complete drug reference 35th edition: The Royal Pharmaceutical Society of Great Britain, London. Electronic version.
WHO (2020). Diabetes and management of Type 2 diabetes. https://www.who.int/publicationsdetail-redirect/who-ncn-ncd-20. (Accessed 23.02.21).
Christensen MMH, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H and Brosen K (2011). The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenetics and genomics 21(12): 837-850.
Boucaud-Maitre D, Ropers B, Porokhov JJ, Altman B, Bouhanick J, Doucet E, Girardin E, Kaloustian VL, Vague J and Emmerich L (2016). Relationship between metformin levels, lactate concentration and mortality. Diabetic Medicine 33(11): 1536-1543.
Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR, Williams KM and Day RO (2013). Population pharmacokinetics of metformin in healthy subjects and patients with Type 2 diabetes mellitus: Simulation of doses according to renal function. Clinical Pharmacokinetics 52: 373-384. 10.1007/s40262-013-0046-9.
Porta V, Schramm VSG, Kano EK, Koono EE, Armando YP, Fukuda K and Serra CH (2008). HPLC-UV determination of metformin in human plasma for application in pharmacokinetics and bioequivalence studies. Journal of Pharmaceutical and Biomedical Analysis 46 (1): 143 - 147.10.1016/j.jpba.2007.10.007.
Uçaktürk E (2013). The development and validation of a gas chromatography-mass spectrometry method for the determination of metformin in human plasma. Analytical Methods 5 (18): 4723-30. 10.1039/C3AY40507A.
Harahap Y, Dianpratami K, Wulandari M and Rahmawati R (2012). Bioequivalence study of metformin HCl XR caplet formulations in healthy Indonesian volunteers. Journal of Life Science 6: 20- 27. 10.4172/jbb.1000051.
Chhetri HP, Thapa P and Schepdael AV (2014). Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharmaceutical Journal 22 (5): 483-487. 10.1016/j.jsps.2013.12.011.
Meyers DR, DeFehr J, Bennett WM, Porter GA and Olsen GD (1978). Gentamicin binding to serum and plasma proteins. Clinical Pharmacology and Therapy 23: 356-60.
Montoya-Eguía SI, Garza-Ocañas DD, BadilloCastañeda T, Tamez-de la OE, Zanatta-Calderón T and Gomez-Meza MV (2015). Comparative pharmacokinetic study among 3 metformin formulations in healthy mexican volunteers: A single-dose, randomized, open-label, 3-period crossover study. Current Therapeutic Research 77: 18 - 23.
Nakamaru Y, Hayashi Y, Martin D, Heuer H, Noriko H and Akimoto K (2015). Investigation of potential pharmacokinetic interactions between teneligliptin and metformin in steady-state conditions in healthy adults. Clinical Therapeutics 37(9): 2007 - 2018.
International Conference on Harmonization (2006). "Q29(R1): Text on Validation of Analytical Procedures". Federal register CPMP/ICH/381/95.
Murthy TGK and Geethanjali J (2014). Development of a validated RP-HPLC method for simultaneous estimation of metformin hydrochloride and rosuvastatin calcium in bulk and In-house formulation. Journal of Chromatography and Separation Techniques 5 (6): 1-7. 10.4172/2157-7064.1000252.
Harvey D (2000). Modern analytical chemistry. The McGraw-Hill Company. Inc. University of California, United States Pp 39.
Pawan PK and Gokul ST (2017). Development of validated HPLC-UV method for simultaneous determination of metformin, amlodipine, Glibenclamide and atorvastatin in human plasma and application to protein binding. Bulletin of Faculty of Pharmacy, Cairo University 55: 129-139.
Saeed-Arayne M, Sultana N and Zuberi HM (2006). Development and validation of RP-HPLC method for analysis of metformin. Pakistan Journal of Pharmaceutical Sciences 19(3): 231-235.