Hepato-protective activity of ethanol extract of Moringa oleifera leaves in acute rifampicin-induced hepatotoxicity Activité hépatoprotectrice de l'extrait éthanolique de feuilles de <i>Moringa oleifera</i> dans l'hépatotoxicité aiguë induite par la rifampicine
Main Article Content
Abstract
ENGLISH
Background: Drugs are an important cause of liver injury. The use of natural remedies for the treatment of liver diseases has a long history, and medicinal plants are still used all over the world for this purpose. For their medicinal and nutritional values, the Moringa oleifera plant is of particular interest.
Objective: In the current study, we aimed to explore the hepato-protective effects of ethanol extract of Moringa oleifera leaves in acute rifampicin-induced hepatotoxicity.
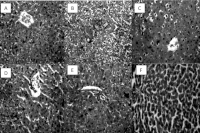
Methods: Thirty mice (18-20 g) were randomly assigned into 6 groups (n = 5). Group I received normal saline only; groups II, III, IV, V, and VI were administered with single oral rifampicin 400 mg/kg, treated with normal saline, different doses of ethanol extract of Moringa oleifera (200, 400, and 800 mg/kg), and ascorbic acid, respectively. Serum levels of liver enzymes Alanine transaminase (ALT), Aspartate transaminase (AST), Alkaline phosphatase (ALP), catalase and superoxide dismutase (SOD) were measured in the serum. Furthermore, phytochemical constituents were also measured in the Moringa oleifera leaves extract.
Results: Treatment with an ethanol extract of Moringa oleifera decreased liver enzyme levels and improved oxidative stress status in hepatotoxic mice in a dose-dependent manner.
Conclusion: Normal levels of liver enzymes, an increase in total antioxidant capacity, and a decrease in lipid peroxidation level uncovered the hepatoprotective effects of the ethanol extract of Moringa oleifera. It seems the antioxidant and hepatoprotective effects of botanical extracts are basically linked with their phenol and flavonoid properties that neutralize oxidant agents. However, more studies are required to implement our strategy.
FRENCH
Contexte: Les médicaments sont une cause importante de lésions hépatiques. L'utilisation de remèdes naturels pour le traitement des maladies du foie a une longue histoire et les plantes médicinales sont encore utilisées à cette fin dans le monde entier. Pour ses valeurs médicinales et nutritionnelles, la plante Moringa oleifera présente un intérêt particulier.
Objectif: Dans la présente étude, nous avons cherché à explorer les effets hépatoprotecteurs de l'extrait éthanolique de feuilles de Moringa oleifera dans l'hépatotoxicité aiguë induite par la rifampicine.
Méthodes: Trente souris (18-20 g) ont été réparties au hasard en 6 groupes (n = 5). Le groupe I a reçu uniquement une solution saline normale ; les groupes II, III, IV, V et VI ont reçu une seule dose orale de rifampicine de 400 mg/kg, traités avec une solution saline normale, différentes doses d'extrait éthanolique de Moringa oleifera (200, 400 et 800 mg/kg) et de l'acide ascorbique, respectivement. Les taux sériques des enzymes hépatiques alanine transaminase, aspartate transaminase, lactate déshydrogénase et phosphatase alcaline, glutathion réduit (GSH), catalase, superoxyde dismutase et peroxydation lipidique sont mesurés dans le tissu hépatique à l'aide de kits spécifiques à la fin de la période expérimentale. En outre, les constituants phytochimiques ont également été mesurés dans l'extrait de feuille de Moringa oleifera.
Résultats: Le traitement avec un extrait éthanolique de Moringa oleifera a diminué les niveaux d'enzymes hépatiques et amélioré l'état de stress oxydatif chez des souris hépatotoxiques de manière dose-dépendante.
Conclusion: Des niveaux normaux d'enzymes hépatiques, une augmentation de la capacité antioxydante totale et une diminution du niveau de peroxydation lipidique ont mis en évidence les effets hépatoprotecteurs de l'extrait éthanolique de Moringa oleifera. Il semble que les effets antioxydants et hépatoprotecteurs des extraits botaniques soient essentiellement liés à leurs propriétés phénoliques et flavonoïdes qui neutralisent les agents oxydants. Cependant, des études supplémentaires sont nécessaires pour mettre en œuvre notre stratégie.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
1. Kim J, Nam WS, Kim S J, Kwon OK, Seung EJ, Jo JJ, Shresha R, Lee TH, Jeon TW, Ki SH, Lee HS, Lee S (2017). Mechanism Investigation of Rifampicin-Induced Liver Injur y Using Comparative Toxicoproteomics in Mice. International Journal of Molecular Sciences 18(7): 1417. doi: 10.3390/ijms18071417.
2. Ranganathan V, Punniamurthy N, Basheer Ahamad D, Sathesh Kumar S (2020). Evaluation of Hepatoprotective activity of Moringa oleifera in chicken. The Journal of Phytopharmacology 9(3): 175-177.
3. World Health Organization Tuberculosis, (2017). Fact Sheet No. 104, Available online: http://www.who.int/mediacentre/factsheets/fs104/en/.
4. World Health Organization, (2010). Treatment of Tuberculosis Guidelines. 4th ed. World Health Organization; Geneva, Switzerland: 2010. p. 46. [Google Scholar].
5. Girling DJ (1977). Adverse reactions to rifampicin in antituberculosis regimens. Journal of Antimicrobial Chemotherapy 3:115-132. doi:10.1093/jac/3.2.115. [PubMed] [CrossRef] [Google Scholar].
6. Capelle P, Dhumeaux D, Mora M, Feldmann G, Berthelot P (1972). Effect of rifampicin on liver function in man. Gut 13:366-371. doi:
10.11.36/gut.13.5.366 [PMC free article] [PubMed] [CrossRef] [Google Scholar].
7. Westphal J, Vetter D, Brogard J (1994). Hepatic side-effects of antibiotics. Journal of Antimicrobial Chemotherapy 33:387-401. doi:
10.1093/jac/33.3.387. [PubMed] [CrossRef] [Google Scholar].
8. Ganie SA, Zargar BA, Masood A, Zargar MA (2013). Hepatoprotective and antioxidant activity of rhizome of Podophyllum hexandrum against carbon tetra chloride induced hepatotoxicity in rats. Biomedical and Environmental Sciences 6(3):209- 221.
9. Mamat SS, Kamarolzaman MF, Yahya F, Mahmood ND, Shahril MS, Jakius KF (2013). Methanol extract of Melastoma malabathricum leaves exerted antioxidant and liver protective activity in rats. BMC Complementary and Alternative Medicine 13(1):326.
10. Rivas R, Oliveira MT, Santos MG (2013). Three cycles of water deficit from seed to young plants of Moringa oleifera woody species improves stress tolerance. Plant Physiology and Biochemistry 63:200-8.
11. Rivas-San Vicente M, Plasencia J (2011). Salicylic acid beyond defence: Its role in plant growth and development. Journal of Experimental Botany 62:3321-38.
12. Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and
pharmacology of Moringa oleifera leaves: an overview. International Journal of Molecular Sciences 16:12791-835.
13. Cuellar-Nunez ML, Luzardo-Ocampo I, Campos-Vega R, Gallegos-Corona MA, Gonzalez de Mejia E, Loarca- Pina G (2018). Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Research International 105:159-168.
14. Dollah S, Abdulkarim SM, Ahmad SH, Khoramnia A, Mohd Ghazali H (2016). Physicochemical properties of Moringa oleifera seed oil enzymatically interesterified with palm stearin and palm kernel oil and its potential application in food. Journal of the Science of Food and Agriculture 96 (10): 3321-3333.
15. Kou X, Li B, Olayanju JB, Drake JM, Chen N (2018). Nutraceutical or pharmacological potential of Moringa oleifera lam, Nutrients 10 (3):343. Doi: 10.3390/nu10039343.
16. Qamar H, Rehman S, Chauhan DK (2019). Current status and future perspective for research on medicinal plants with anticancerous activity and minimum cytotoxic value. Current Drug Targets 20 (12):1227-1243.
17. Trease GE, Evan WC (2002). Textbook of Pharmacognosy. 15th Edn. London: Saunders Publishers.229:393.
18. De Silva GO, Abeysundara AT, Aponso MM (2017). Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. American Journal of Essential Oils and Natural Products 5:29-32.
19. Liu Y, Kim HJ (2017). Fourier transform infrared spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors 17:1469.
20. Bolade OP, Akinsiku AA, Adeyemi AO, Williams AB, Benson NU (2018). Dataset on phytochemical screening, FTIR and GC-MS characterization of Azadirachta indica and Cymbopogon citratus as reducing and stabilizing agents for nano-particles synthesis. Data in Brief 20: 917-926.
21. Rajesh M, Latha M (2004). Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. Journal of Ethnopharmacology 91(1):99-104.
22. Mitchell J, Jollow D, Potter W, Davis D, Gillette J, Brodie B (1973). Acetaminophen induced hepatic necrosis. I. Role of drug metabolism. Journal of Pharmacology and Experimental Therapeutics 187(1):185-94.
23. Moossavi M, Hoshyar R, Hemmati, M, Farahi A, Javdani H (2016). An in vivo study on the hepato-protective effects of Crocus sativus, Ziziphus jujuba and Berberis vulgaris against acute acetaminophen and rifampicin-induced hepatotoxicity. Clinical Phytoscience 2:16. DOI 10.1186/s40816-016-0030-7.
24. Olaleye MT, Rocha BJ (2008). Acetaminophen-induced liver damage in mice: effects of some medicinal plants on the oxidative defense system. Experimental and Toxicology Pathology 59(5):319-27.
25. Abd elhameed MF, Salama AAA, Attia TM, Elbatran SA, Ismaeil IE, Hassan A (2018). Protective Effects of Moringa oleifera extract on Isoniazid and Rifampicin Induced Hepatotoxicity in Rats: Involvement of Adiponectin and Tumor Necrosis Factor-α. Egyptian Journal of Veterinary Sciences 2349.1025
26. Younis YN, Khan MI, Zahoor TZT, Faisal MN (2022). Phytochemical and antioxidant screening of Moringa oleifera for its utilization in the management of hepatic injury. Frontier in Nutrition 9:2022. https://doi.org/10.3389/fnut.2022.1078896.
27. Saki M, De Villiers H, Ntsapi C, Tiloke C (2023). The Hepatoprotective Effects of Moringa oleifera against Antiretroviral-Induced Cytotoxicity in HepG2 Cells: A Review. Plants 12(18): 3235.DOI: 10.3390/plants12183235.
28. Altaee RA, Fadheel QJ (2023). The nephroprotective effects of Moringa oleifera extract against contrast induced nephrotoxicity.
Journal of Pharmaceutical Research International 33:63-70. Doi:10.9734/jpri/2021/v33i22A31389. [CrossRef] [Google Scholar].
29. Al-sultan M, Al-sowayan N (2024). The Protective Effect of Moringa oleifera leaves Extract on Paracetamol Hepatotoxicity in Male Rats. Journal of Biomedical Science and Engineering 17:72-82. Doi: 10.4236/jbise.2024.173006.
30. Abd-Elnaib YA, Elsayed IE, AbdEldaim MA (2022). Anti-inflammatory and antioxidant effect of Moringa oleifera against bisphenol-A- induced hepatotoxicity. Egyptian Liver Journal 12:57(2022). https://doi.org/10.1186/s43066-022-00219-7.
31. Termentzi A, Kokkalou E (2008). LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Medica 74(5):573-81.
32. Goli SAH, Barzegar M, Sahari MA (2004). Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chemistry 92(3):521-525. DOI: 10.1016/j.foodchem.2004.08.020.