Evaluation of antioxidant and antibacterial activities of extracts of Celtis philippensis Blanco (Ulmaceae) on multidrug resistant wound pathogens
Main Article Content
Abstract
Background: Antimicrobial resistance among wound pathogens is increasing, creating the need to search for new and effective agents, especially among medicinal plants used to treat wounds traditionally. There is a worldwide search for medicinal plants with good antibacterial and antioxidant activities to treat infected wounds.
Objectives: Celtis philippensis used locally to treat infections was evaluated for its antioxidant and antibacterial activities against multidrug resistant (MDR) pathogens from infected wounds from three Nigerian hospitals.
Methods: Methanol extract of C. philippensis stem was tested at 20 and 10mg/ml on 32 wound pathogens comprising of Staphylococcus aureus (14), Pseudomonas aeruginosa (10) and Klebsiella pneumoniae (8). Agar diffusion methods was used to determine antibiogram and susceptibility to extracts while agar dilution method was used to determine minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC). The antioxidant potential was evaluated by four methods: catalase activity, lipid peroxidation inhibition, hydrogen peroxide radical scavenging activity and DPPH radical scavenging activity. Statistical analysis was done using ANOVA.
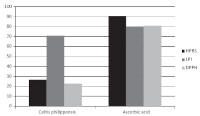
Results: Antibiogram showed that the test organisms were MDR bacteria. The extract was active on all the tested pathogens with zones of inhibition of between 13-35mm. The MIC and MBC values ranged between 0.625- 5mg/ml for all the test organisms and MIC index indicated bactericidal activity. The methanol extract showed good antioxidant activity (lipid peroxidation inhibition and catalase activity) comparable with ascorbic acid standard.
Conclusion: Antioxidant and antibacterial activities of Celtis philippensis justified its folkloric use in treating infections. Moreover, good activity on multidrug resistant wound pathogens highlights its potentials as a source of antimicrobial agents to treat wounds infected with MDR pathogens.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
Kumar MS, Sripriya R, Raghavan HV, Sehgal PK (2006). Wound healing potential of Cassia fistula on infected albino rat model. Journal of Surgical Research 131: 283-289.
Akingbade OA, Akinjinmi AA, Ezechukwu US, Okerentugba PO, Nwanze JC, Onoh CC, Okonko IO (2013). Antibacterial effect of Moringa oleifera on multidrug resistant Pseudomonas aeruginosa isolates from wound infections in Abeokuta, Ogun State, Nigeria. World Rural Observation 5(3):6-10.
Bowler P, Duerden B and Armstrong D (2001). Wound microbiology and associated approaches to wound management. Clinical Microbiology Review 14(2): 244-69.
Posluszny J, Conrad P and Marcia H (2011). Surgical burn wound infections and their clinical implications. Journal of Burn Care Research 32(2): 324-33.
Motayo BO, Akinbo JA, Ogiogwa IJ, Idowu AA, Nwanze JC, Onoh CC and Okerentugba PO (2013). Innocent-Adiele H. C, Okonko, I. O. Bacteria Colonisation and Antibiotic Susceptibility Pattern of Wound Infections in a Hospital in Abeokuta. Frontiers in Science 3 (1): 43-48.
Oladeinde BH, Omoregie R, Olley M, Anunibe JA and Onifade AA (2012). A 5 - year surveillance of wound infections at a rural tertiary hospital in Nigeria. African Health Science 13 (2): 351-356.
Akingbade OA, Balogun SA, Ojo DA, Afolabi RO, Motayo BO, Okerentugba PO and Okonko IO (2012). Plasmid Profile Analysis of Multidrug Resistant Pseudomonas aeruginosa Isolated from Wound Infections in South West, Nigeria. World Applied Sciences Journal 20(6): 766-775.
Okeke IN, Lamikanra A and Edelman R (1996). Socioeconomic and behavioural factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerging Infectious Diseases 5: 18-27.
Archana SJ, Paul R and Tiwari A (2011). Indian medicinal plants: A rich source of natural immunemodulator. International Journal of Pharmacology 7 (2): 198-205.
Mahima RA, Deb R, Latheef S and Samad HA (2012). Immunomodulatory and therapeuticpotentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pak.J.Biol.Sci. 15: 754-774.
Dong-Ping X, Ya L, Xiao M, Tong Z, Yue Z, Jie Z, JiaoJiao Z and Hua-Bin L (2017). Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. International Journal of Molecular Science 18(1): 96
Kumar B, Vijayakumar M, Govindarajan R and Pushpangadan P (2007). Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. Journal of Ethnopharmacology 114: 103-113
Keay, R.W.J. (1989). Trees of Nigeria. Oxford University Press. Pg 282-287
El-Alfy TS, El-Gohary HMA, Sokkar NM, Horsny M and Al-Mahdy DA (2011). A new flavonoid CGlycoside from Celtis australis L. and Celtis
occidentalis L. leaves and potential antioxidant and cytotoxic activities. Scientia Pharmacetica 79(4): 963-975.
Perez C, Pauli M and Bazerque P (1990. An antibiotic assay by agar-well diffusion method. Acta Biologiae et Medicinae Experimentalis 15: 113-115
Cheesebrough, M (2000). District laboratory practice in tropical countries Part 2. UK: Cambridge University Press. 134-143
Aebi H (1984). Catalase. In: Methods of Enzymatic Analysis, Bergmeyer, H.U. (Ed.). Academic Press Inc., New York, USA. 2: 673-685.
Wettasinghe M and Shahidi F (2000). Scavenging of reactive oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chemistry 70 (1): 17-26.
Sowndharrajan K, Siddhuraju P and Manian S (2010). In vitro evaluation of the antioxidant activities in the differentially processed seeds from underutilized legume, Bauhina vahlii Wight & Arn. Food Science and Biotechnology 19: 503-509.
Saha K, Lajis NH, Israf DA, Hamzah AS, Khozirah S, Khamis S, Syahida A (2004). Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. Journal of Ethnopharmacology 92(2-3): 263-267.
Clinical Laboratory Standard Institute [CLSI] (2007). Performance Standard for Antimicrobial Susceptibility Testing. Seventeenth Informational Supplement. M100-S17. Wayne, Pennysylvania, USA.
Ahmed FY, Farghaly Aly U, Abd El-Baky RM and Waly NGFM (2020). Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on MDR Pseudomonas aeruginosa Strains. International Journal of Nanomedicine 15: 3393-3404.
Diab M, Fam N, El-said M, El-dabaa E, El Defrawy I and Saber M (2013). Occurrence of VIM-2 metallo-?-lactamases in imipenem resistant and susceptible Pseudomonas aeruginosa clinical isolates from Egypt. African Journal of Microbiology Research 7(35): 4465-4472.
Dou JL, Jiang YW, Xie JQ and Zhang XG (2016). New Is Old, and Old Is New: Recent Advances in AntibioticBased, Antibiotic-Free and Ethnomedical Treatments against Methicillin-Resistant Staphylococcus aureus Wound Infections. International Journal of Molecular Science 17(5): 617.
King James Bible (2000). The Zondervan Corporation. 2Kings 20(7): pp 388
Rabe T and Van Staden J (1997). Antibacterial activity of South African plants used for medicinal purposes. Journal of Ethnopharmacology 56: 81-87.
Vlietinck AJ, Van Hoof L, Totte J, Lasure A, Vanden Berghe D, Rwangobo PC and Mvukiyuniwami J (1995). Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. Journal of Ethnopharmacology 46: 31-47.
Mamidou Koné W, Kamanzi Atindehou K, KacouN'Douba A and Dosso M (2007). Evaluation of 17 medicinal plants from northern Côte d'ivoire for their in vitro activity against Streptococcus pneumoniae. African Journal of Traditional, Complementary and Alternative Medicines 4(1): 17-22.
Dudonneì S, Vitrac X, CoutieÌre P, Woillez M and Meìrillon JM (2009). Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry 57:1768-1774