Comparative quality assessment of selected fluconazole brands in Ibadan, Nigeria Évaluation comparative de la qualité de certaines marques de fluconazole à Ibadan, au Nigéria
Main Article Content
Abstract
ENGLISH
Background: Drug therapeutic failure is a significant challenge currently ravaging the healthcare system of developing countries like Nigeria. This is often linked to the variable quality of generic drug brands. Fluconazole, a triazole antifungal agent, is of clinical importance due to its excellent penetrability in the CSF.
Objective: To evaluate the quality and pharmaceutical equivalence between generic brands of fluconazole and the innovator brand that might affect its performance and interchangeability within Ibadan, Nigeria.
Methods: Seventeen brands of fluconazole including the innovator brand were evaluated for their physicochemical properties (weight uniformity), mechanical properties (crushing strength and friability), release properties (disintegration and dissolution time tests), content assay, and antifungal activity. Statistical analysis was conducted using ANOVA (p < 0.05) to assess significant differences.
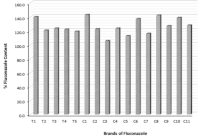
Results: All brands passed the weight uniformity test, but only three passed the friability test. One brand from the tablets and the capsules failed the disintegration time test. Notably, only one brand passed the content assay test. The tablets released > 80 % of fluconazole within 30 min while all the capsules including the innovator brand exhibited a burst release. Two brands showed no activity against Candida albicans. The innovator brand had a significantly higher antifungal activity (p < 0.05) than the generics.
Conclusion: The differences in quality among the brands suggest that none would adequately substitute the innovator brand.
FRENCH
Contexte: L'échec thérapeutique des médicaments est un défi important qui ravage actuellement le système de santé des pays en développement comme le Nigéria. Ce problème est souvent lié à la qualité variable des marques de médicaments génériques. Le fluconazole, un agent antifongique triazole, est d'une importance clinique en raison de son excellente pénétration dans le LCR.
Objectif: Évaluer la qualité et l'équivalence pharmaceutique entre les marques génériques de fluconazole et la marque innovante qui pourraient affecter ses performances et son interchangeabilité à Ibadan, au Nigéria.
Méthodes: Dix-sept marques de fluconazole, dont la marque innovante, ont été évaluées pour leurs propriétés physicochimiques (uniformité du poids), leurs propriétés mécaniques (résistance à l'écrasement et friabilité), leurs propriétés de libération (tests de désintégration et de temps de dissolution), leur dosage et leur activité antifongique. Une analyse statistique a été réalisée à l'aide de l'ANOVA (p < 0,05) pour évaluer les différences significatives.
Résultats: Toutes les marques ont passé avec succès le test d'uniformité du poids, mais seulement trois ont réussi le test de friabilité. Une marque de comprimés et de gélules a échoué au test de temps de désintégration. Il convient de noter qu'une seule marque a réussi au test d'analyse du contenu. Les comprimés ont libéré plus de 80% de fluconazole en 30 minutes, tandis que toutes les gélules, y compris la marque innovante, ont présenté une libération en rafale. Deux marques n'ont montré aucune activité contre Candida albicans. La marque innovante avait une activité antifongique significativement plus élevée (p < 0,05) que les génériques.
Conclusion: Les différences de qualité entre les marques suggèrent qu'aucune ne pourrait remplacer adéquatement la marque innovante.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
1. Desai RJ, Sarpatwari A, Dejene S, Khan NF, Lii J, Rogers JR, et al. Differences in rates of switchbacks after switching from branded to authorized generic and branded to generic drug products: cohort study. British Medical Journal. 2018;361.
2. Al Ali L, Jagal J, Joseph J, Ahmed IS, Rawas-Qalaji M. Pharmaceutical equivalency of locally and regionally manufactured generic pharmaceutical products in UAE. Saudi Pharmaceutical Journal. 2022;30(9):1243-51.
3. Ndichu ET, Ohiri K, Sekoni O, Makinde O, Schulman K. Evaluating the quality of antihypertensive drugs in Lagos State, Nigeria. PloS one.
2019;14(2):e0211567.
4. Bunu J, Alfred-Ugbenbo D, Dode E, Lambert F. In vitro bioequivalence analysis of some Artemether- Lumefantrine-based combination Formulations utilized in Nigeria. Modern Approaches in Pharmacy & Pharmaceutical Sciences. 2023;1(1):23-9.
5. Awofisayo SO, Awofisayo OA, Eyen N, Udoh IE. Comparative assessment of the quality control measurements of multisource ofloxacin tablets marketed in Nigeria. Dissolution technologies. 2010;17:20-5.
6. Oli AN, Ibeabuchi MU, Enweani IB, Emencheta SC. Pharmaceutical Quality of Selected Metronidazole and Ciprofloxacin Infusions Marketed in South Eastern Nigeria. Drug, Healthcare and Patient Safety. 2020;12:103-12.
7. Nwachukwu N, Ubieko EA. In vitro quality assessment of ten brands of metronidazole benzoate suspensions marketed in Warri, Nigeria.
GSC Biological Pharmaceutical Sciences 2020;13(2):190-8.
8. Igboasoiyi AC, Egeolu AP, Edet EM. Quality evaluation and UV spectrophotometric assay of ten brands of amlodipine tablets marketed in Uyo, Nigeria. Journal of Pharmacy & Bioresources. 2020;17(1):60-5.
9. Hassan IA, Adegbola AJ, Soyinka JO, Onyeji CO, Bolaji OO. Post-Marketing Surveillance of Quality of Artemether Injection Marketed in Southwest Nigeria. The American Journal of Tropical Medicine and Hygiene. 2020;103(3):1258-65.
10. Abu-Saeed K, Muslim JO. Chemical and microbial analysis of different brands of Ibuprofen solid dosage formulations sold in Ilorin, Kwara State. West African Journal of Pharmacy. 2023;27(1):135-44.
11. Budani MC, Fensore S, Di Marzio M, Tiboni GM. Maternal use of fluconazole and congenital malformations in the progeny: A meta-analysis of the literature. Reproductive Toxicology (Elmsford, NY). 2021;100:42-51.
12. Choy HL, Gaylord EA, Doering TL. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio. 2023;14(4):e0135323.
13. Corrêa JCR, Duarte Vianna-Soares C, Salgado HRN. Development and validation of dissolution test for fluconazole capsules by HPLC and derivative UV spectrophotometry. J Chromatography Research International. 2012;2012.
14. Odeniyi MA, Adegoke OA, Adereti RB, Odeku OA, Itiola OA. Comparative analysis of eight brands of sulfadoxine-pyrimethamine tablets. Tropical Journal of Pharmaceutical Research. 2003;2(1):161-7.
15. British Pharmacopoeia C. British pharmacopoeia 2014. London: Stationery Office; 2014.
16. Pharmacopeia US. USP 43 NF 38. USA: United States Pharmacopeial Convention; 2020.
17. Nakamura K, Kambayashi A, Onoue S. Quantitative assessment of disintegration rate is important for predicting the oral absorption of solid dosage forms containing poorly soluble weak base drugs. European Journal of Pharmaceutics and biopharmaceutics: official journal of
Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2022;180:23-32.
18. Friedel HD, Brown CK, Barker AR, Buhse LF, Keitel S, Kraemer J, et al. FIP Guidelines for Dissolution Testing of Solid Oral Products. Journal Pharmaceutical Sciences. 2018;107(12):2995-3002.