Adverse drug reactions to first-line anti-tuberculosis drugs in a chest hospital, Ibadan
Main Article Content
Abstract
Background: Patients treated for TB frequently experience adverse drug reactions caused by multiple medications taken for long duration. These reactions have the potential of causing poor adherence, treatment failure, relapse or emergence of drug resistance. Preventing or minimizing such reactions can improve patient's adherence and treatment success rate.
Objectives: This study assessed the incidence and risk factors for adverse drug reactions (ADRs) to first line anti-TB drugs among patients treated for drug susceptible TB at Government Chest Hospital, Jericho Ibadan.
Methods: This was a cross sectional retrospective study. Data from medical records of patients treated between January and December 2018 was retrieved and analyzed with SPSS statistical package. This study was approved by the Research Ethics Review Committee of Oyo State Ministry of Health.
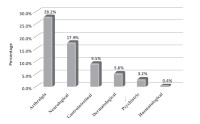
Results: Case files of 252 patients were included in the study. Overall incidence of ADRs was 49.6%. Majority of the patients had only one type (32.5%) of ADRs and the most common was arthralgia (28.2%,). Risk factors for ADRs among patients include age, female gender and comorbidities.
Conclusion: About half of the patients experienced ADRs during TB treatment and major risk factors identified were age, female gender and presence of comorbidities.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
World Health Organisation. (2018). Global tuberculosis report 2018. France: World Health Organization. Available at:
https://apps.who.int/iris/handle/10665/274453. Accessed May 13, 2019.
Holmes KK, Bertozzi S, Bloom BR, Jha P. (2017). Tuberculosis--Major Infectious Diseases. The International Bank for Reconstruction and
Development/The World Bank.
Sunday O, Oladimeji O, Ebenezer F, Akintunde B, Abiola TO, Saliu A, Abiodun O (2014). Treatment outcome of tuberculosis patients registered at DOTS centre in Ogbomoso, Southwestern Nigeria: a 4-year retrospective study. Tuberculosis research and treatment, 2014.
Xia YY, Liu FY, Wang XM, Yuan YL, Chen YX, Zhou L, Zhan S (2010). Design of the anti-tuberculosis drugs induced adverse reactions in China National Tuberculosis Prevention and Control Scheme Study (ADACS). BMC public health 10(1): 267.
Wang PY, Xie SY, Hao Q, Zhang C, Jiang BF (2012). NAT2 polymorphisms and susceptibility to antituberculosis drug-induced liver injury: a metaanalysis. The International journal of tuberculosis and lung disease 16(5): 589-595.
Farazi A, Sofian M, Jabbariasl M, Keshavarz S. (2014). Adverse reactions to anti-tuberculosis drugs in Iranian tuberculosis patients. Tuberculosis research and treatment, 2014.
Horsburgh CR, Barry III CE, Lange C. (2015). Treatment of tuberculosis. New England Journal of Medicine, 373(22): 2149-2160.
Tostmann A, Boeree MJ, Aarnoutse RE, De Lange WC, Van Der Ven AJ, Dekhuijzen R (2008). Antituberculosis drug?induced hepatotoxicity:
concise up? to? date review. Journal of gastroenterology and hepatology 23(2): 192-202.
Hinderaker SG, Ysykeeva J, Veen J, Enarson DA. (2009). Serious adverse reactions in a tuberculosis programme setting in Kyrgyzstan. International Journal of Tuberculosis & Lung Disease 13(12): 1560-1562.
Ibrahim LM, Hadejia IS, Nguku P, Dankoli R, Waziri NE, Akhimien MO, Nsubuga P (2014). Factors associated with interruption of treatment among Pulmonary Tuberculosis patients in Plateau State, Nigeria. Pan African Medical Journal 17(1).
Yang M, Pan H, Lu L, He X, Chen H Tao B, Tang S (2019). Home-based Anti-Tuberculosis Treatment Adverse Reactions (HATTAR) study: a protocol for a prospective observational study. BMJ open 9(3).
Gholami K, Kamali E, Hajiabdolbaghi M, Shalviri G. (2006). Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharmacy practice 4(3): 134.
Damasceno GS, Guaraldo L, Engstrom EM, Theme Filha MM (2013). Adverse reactions to anti tuberculosis drugs in Manguinhos. Rio de Janeiro Brazil. Clinics 68(3): 329-37.
Przybylski G, D?browska A, Trzci?ska H. (2014). Alcoholism and other socio-demographic risk factors for adverse TB-drug reactions and
unsuccessful tuberculosis treatment-data from ten years' observation at the Regional Centre of Pulmonology, Bydgoszcz, Poland. Medical science monitor: international medical journal of experimental and clinical research 20: 444.
Zhang T, Du J, Yin X, Xue F, Liu Y, Li R, Luo C, Li L, Li X (2016). Adverse events in treating smear-positive tuberculosis patients in China. International journal of environmental research and public health 13(1): 86.
Dosumu EA (2002). Side-effects of drugs used in directly observed treatment short-course in newly diagnosed pulmonary tuberculosis subjects in Nigerians: a controlled clinical study. The Nigerian Postgraduate Medical Journal 9(1): 34-37.
Michael OS, Sogaolu OM, Fehintola FA, Ige OM, Falade CO (2016). Adverse events to first line antituberculosis drugs in patients co-infected with HIV and Tuberculosis. Annals of Ibadan Postgraduate Medicine 14(1).
Singla R, Sharma SK, Mohan A, Makharia G, Sreenivas V, Singh S. (2010). Evaluation of risk factors for antituberculosis treatment induced
hepatotoxicity. Indian Journal of Medical Research 132(1): 81-87.
Ali AH, Belachew T, Yami A, Ayen WY. (2013). Antituberculosis drug induced hepatotoxicity among TB/HIV co-infected patients at Jimma University Hospital, Ethiopia: nested case-control study. PLoS One 8(5): e64622.
Xiang Y, Ma L, Wu W, Liu W, Li Y, Zhu X, Wang Q,(2014). The incidence of liver injury in Uyghur patients treated for TB in Xinjiang Uyghur
autonomous region, China, and its association with hepatic enzyme polymorphisms nat2, cyp2e1, gstm1 and gstt1. PloS one 9(1): e85905.
Tweed CD, Wills GH, Crook AM, Dawson R, Diacon AH, Louw CE (2018). Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC medicine 16(1): 46.
Chung-Delgado K, Revilla-Montag A, Guillen-Bravo S, Velez-Segovia E, Soria-Montoya A (2011). Factors associated with anti-tuberculosis medication adverse effects: a case-control study in Lima, Peru. PloS one 6(11): e27610.
Oshi DC, Oshi SN, Alobu I, Ukwaja KN (2014). Profile and treatment outcomes of tuberculosis in the elderly in southeastern Nigeria, 2011-2012. PLoS One 9(11): e111910.