Unraveling the anxiolytic and antidepressant potential of Chromolaena odorata extract: a preclinical investigation Exploration du potentiel anxiolytique et antidépresseur de l'extrait de <i>Chromolaena odorata</i>: une étude préclinique
Main Article Content
Abstract
ENGLISH
Background: Chromolaena odorata Linn (L.) (Asteraceae) is a medicinal plant traditionally used to treat various ailments, including anxiety and depression. However, its neuropharmacological properties have not been extensively studied.
Objective: This study aimed to investigate the anxiolytic and antidepressant effects of C. odorata extract and explore its possible mechanism of action.
Methods: Acute oral toxicity testing was performed following OECD guidelines, and qualitative phytochemical analysis was carried out using standardized procedures. The anxiolytic effects of C. odorata extract were evaluated using the elevated plus maze (EPM) test, while its antidepressant activity was evaluated through the tail suspension test (TST). The extract was administered to mice at doses of 100, 200, and 400 mg/kg, with behavioral parameters carefully recorded over a five-minute observation period. At the fourteen day experimental period, the animals were euthanized using ethyl ether, and brain samples were collected to analyze serotonin and noradrenaline levels, and to evaluate In-vivo brain antioxidant activity.
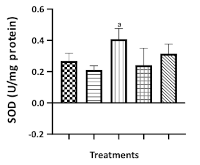
Results: The result revealed that the LD50 of C. odorata extract was greater 2 g/kg. In the Elevated Plus Maze (EPM) test, the extract significantly increased the time spent (P<0.01; 400 mg/kg) and the frequency of exploratory behaviors (P<0.05; 400 mg/kg) in the open arms, while significantly reducing the time spent (P<0.01; 200-400 mg/kg) and the frequency of exploration (P<0.05, 0.01; 200-400 mg/kg) in the closed arms in comparison to the distilled water control group. In the Tail Suspension Test (TST), the extract significantly decreased the duration of immobility (P<0.05-0.001; 100-400 mg/kg) relative to the distilled water treated group. Furthermore, at 400 mg/kg, the extract significantly elevated serotonin (P<0.01) and noradrenaline (P<0.0001) levels compared to the negative control. The extract also demonstrated significant antioxidant activity, enhancing brain antioxidative biomarker levels.
Conclusion: This study highlights the anxiolytic and antidepressant potential of C. odorata extract. Its antidepressant effects may be attributed to increased serotonin and noradrenaline levels, along with the inhibition of oxidative radical generation.
FRENCH
Contexte: Le Chromolaena odorata Linn (L.) (Asteraceae) est une plante médicinale traditionnellement utilisée pour traiter diverses affections, notamment l'anxiété et la dépression. Cependant, ses propriétés neuropharmacologiques n'ont pas fait l'objet d'études approfondies.
Objectif: Cette étude vise à examiner les effets anxiolytiques et antidépresseurs de l'extrait de C. odorata et à explorer son mécanisme d'action possible.
Méthodes: Des tests de toxicité orale aiguë ont été réalisés conformément aux lignes directrices de l'OCDE, et une analyse phytochimique qualitative a été effectuée selon des procédures normalisées. Les effets anxiolytiques de l'extrait de C. odorata ont été évalués à l'aide du test du labyrinthe en croix surélevé (EPM), tandis que son activité antidépressive a été évaluée à l'aide du test de suspension de la queue (TST). L'extrait a été administré à des souris à des doses de 100, 200 et 400 mg/kg, et les paramètres comportementaux ont été soigneusement enregistrés sur une période d'observation de cinq minutes. Au bout de la période expérimentale de quatorze jours, les animaux ont été euthanasiés à l'éther éthylique, et des échantillons de cerveau ont été prélevés pour analyser les taux de sérotonine et de noradrénaline, et pour évaluer l'activité in vivo antioxydante cérébrale.
Résultats: Les résultats ont révélé que la DL50 de l'extrait de C. odorata était supérieure à 2 g/kg. Dans le test du labyrinthe en croix surélevé (EPM), l' extrait a augmenté de manière significative le temps passé (P<0,01 ; 400 mg/kg) et la fréquence des comportements exploratoires (P<0,05 ; 400 mg/kg) dans les bras ouverts, tout en réduisant de manière significative le temps passé (P<0,01 ; 200-400 mg/kg) et la fréquence d'exploration (P<0,05, 0,01 ; 200-400 mg/kg) dans les bras fermés par rapport au groupe témoin à l'eau distillée. Dans le test de suspension de la queue (TST), l'extrait a réduit de manière significative la durée d'immobilité (P<0,05-0,001 ; 100-400 mg/kg) par rapport au groupe traité à l'eau distillée. En outre, à 400 mg/kg, l'extrait a augmenté de manière significative les taux de sérotonine (p<0,01) et de noradrénaline (p<0,0001) par rapport au témoin négatif. L'extrait a également démontré une activité antioxydante significative, augmentant les niveaux de biomarqueurs antioxydants cérébraux.
Conclusion: Cette étude met en évidence le potentiel anxiolytique et antidépresseur de l'extrait de C. odorata. Ses effets antidépresseurs pourraient être attribués à l'augmentation des taux de sérotonine et de noradrénaline, ainsi qu'à l'inhibition de la production de radicaux oxydatifs.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
1. Verma M, Grover S, Tripathy JP, Singh T, Nagaraja SB, Kathirvel S, Singh G, Nehra R (2019). Co-existing non-communicable diseases and mental illnesses amongst the elderly in Punjab, India. European Endocrinology 15 (2):106.
2. Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB (2008). Anxiety disorders and comorbid medical illness. General Hospital Psychiatry 30(3): 208-25.
3. Zareifopoulos N, Bellou A, Spiropoulou A, Spiropoulos K (2019). Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: a narrative review. COPD: Journal of Chronic Obstructive Pulmonary Disease 16(5-6): 406-17.
4. Clark LA, Watson D (2013). Theoretical and empirical issues in differentiating depression from anxiety. In Psychosocial aspects of depression 39-66 Routledge.
5. Hranov LG (2007). Co-morbid anxiety and depression: illumination of a controversy. International Journal of Psychiatry in Clinical Practice 11(3): 171-89.
6. Yang J, Yuan M, Zhang W (2024). The major biogenic amine metabolites in mood disorders. Frontiers in Psychiatry 15:1460631.
7. Nimgampalle M, Chakravarthy H, Sharma S, Shree S, Bhat AR, Pradeepkiran JA, Devanathan V (2023). Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Research Reviews 89:101994. doi: 10.1016/j.arr.2023.101994
8. Jesulola E, Micalos P, Baguley IJ (2018). Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet? Behavioural Brain Research 341: 79-90.
9. Jiang Y, Zou D, Li Y, Gu S, Dong J, Ma X, Xu S, Wang F, Huang JH (2022). Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 15(10): 1203.
10. Bhatt S, Nagappa AN, Patil CR (2020). Role of oxidative stress in depression. Drug discovery today 25(7): 1270-6.
11. Karanikas E, Daskalakis NP, Agorastos A (2021). Oxidative dysregulation in early life stress and posttraumatic stress disorder: a comprehensive review. Brain Sciences 11(6): 723.
12. Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV (2020). Pharmacotherapy of anxiety disorders: current and emerging treatment options. Frontiers in Psychiatry 11:595584.
13. Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, Rosenzweig-Lipson S (2005). Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx 2(4): 590-611.
14. Olawale F, Olofinsan K, Iwaloye O (2022). Biological activities of Chromolaena odorata: A mechanistic review. South African Journal of Botany 144: 44-57.
15. Omeke PO, Obi JO, Orabueze NI, Ike AC (2019). Antibacterial activity of leaf extract of Chromolaena odorata and the effect of its combination with some conventional antibiotics on Pseudomonas aeruginosa isolated from wounds. Journal of Applied Biology and Biotechnology 7(03): 36-40.
16. Adewumi Alabi M, Olusola-Makinde O, Kolawole Oladunmoye M (2020). Evaluation of phytochemical constituents and antibacterial activity of Chromolaena odorata L. leaf extract against selected multidrug resistant bacteria isolated from wounds. South Asian Journal of Research in Microbiology 5(3): 1-9.
17. Taiwo OB, Olajide OA, Soyannwo OO, Makinde JM (2000). Anti-inflammatory, antipyretic and antispasmodic properties of Chromolaena odorata. Pharmaceutical Biology 38(5): 367-70.
18. Murtala AA, Oladapo OE, Aderionla AA, Olooto WE, Soyinka OO, Folarin RO, Oladoja FA, Shonde OO, Osipitan LE, Adegbe EB, Abolarinwa JA (2023). Sub- chronic (ninety days) toxicity study of hydroethanolic leaf extract of Datura stramonium L. in rodents. Clinical Complementary Medicine and Pharmacology 3(3):100090.
19. Shaikh JR, Patil M (2020). Qualitative tests for preliminary phytochemical screening: An overview. International Journal of Chemical Studies 8(2): 603-8.
20. Maheshwaran L, Nadarajah L, Senadeera SP, Ranaweera CB, Chandana AK, Pathirana RN (2024). Phytochemical Testing Methodologies and Principles for Preliminary Screening/Qualitative Testing. Asian Plant Research Journal 12(5): 11-38.
21. Bedi O, Krishan P (2020). Investigations on acute oral toxicity studies of purpurin by application of OECD guideline 423 in rodents. Naunyn-Schmiedeberg's Archives of Pharmacology 393(4): 565-71.
22. Akindele AJ, Ezenwanebe KO, Anunobi CC, Adeyemi OO (2010). Hepatoprotective and in vivo antioxidant effects of Byrsocarpus coccineus Schum. and Thonn.(Connaraceae). Journal of Ethnopharmacology 129(1): 46-52.
23. Rodgers RJ, Dalvi A (1997). Anxiety, defence and the elevated plus-maze. Neuroscience & Biobehavioral Reviews 21(6): 801-10.
24. Murtala AA, Akindele AJ, Oreagba IA (2022). Effects of Costus afer Extract in Mouse Models of Anxiety and Depression and Its Possible Mechanisms of Action. Tropical Journal of Natural Product Research. 6(4): 654-660
25. Murtala AA, Akindele AJ (2020). Anxiolytic-and antidepressant-like activities of hydroethanol leaf extract of Newbouldia laevis (P. Beauv.) Seem. (Bignoniaceae) in mice. Journal of Ethnopharmacology 249 (112420) : 1-8
26. Murtala AA, Oladapo OE, Oladoja FA, Samuel FM, Adedeji OH, Ogunjimi LO, Alabi AA, Olooto WE, Soyinka OO, Faponle AS, Shonde OO, Osipitan LE, Kasumu EO, Joseph OO, Olaniran EO, Olatunji EF (2024). Xylopia parviflora (A. Rich.) Benth. Mitigates anxiety Behavior and Chronic Mild Stress-induced Depression-like Behavior in Mice: The involvement of Biogenic Amine Neurotransmitters, Cyclooxygenase-2 and Stress Biomarkers in its Antidepressant Activity. Pharmacological Research-Modern Chinese Medicine. 13 (100541): 1-11
27. Kudo H, Kokunai T, Kondoh T, Tamaki N, Matsumoto S (1990). Quantitative analysis of glutathione in rat central nervous system: comparison of GSH in infant brain with that in adult brain. Brain Research 511(2): 326-8.
28. Patocková J, Marhol P, TUMOVÁ E, Krsiak M, Rokyta R, Štípek S, Crkovska J, Andél M (2003). Oxidative stress in the brain tissue of laboratory mice with acute post insulin hypoglycemia. Physiological Research 52:131-5.
29. Popov B, Gadjeva V, Valkanov P, Popova S, Tolekova A (2003). Lipid peroxidation, superoxide dismutase and catalase activities in brain tumor tissues. Archives of Physiology and Biochemistry 111(5): 455-9.
30. Sharma S, Pandey J, Jain S, Singh V (2022). Examining Anxiety and Risk-taking in Healthy Male and Female Wistar Rats using Spatial and Temporal Analysis of Elevated Plus Maze. BioRxiv doi: https://doi.org/10.1101/2022.11.16.516842
31. Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AF, Gründemann D, Schömig E, Lesch KP, Gerlach M, Reif A (2009). Decreased anxiety in mice lacking the organic cation transporter 3. Journal of Neural Transmission 116: 689-97.
32. Thippeswamy BS, Mishra B, Veerapur VP, Gupta G (2011). Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety. Indian Journal of Pharmacology 43(1): 50-5.
33. Puech C, Badran M, Runion AR, Barrow MB, Qiao Z, Khalyfa A, Gozal D (2022). Explicit memory, anxiety and depressive like behavior in mice exposed to chronic intermittent hypoxia, sleep fragmentation, or both during the daylight period. Neurobiology of Sleep and Circadian Rhythms 13:100084.
34. Pryce CR, Seifritz E (2011). A translational research framework for enhanced validity of mouse models of psychopathological states in depression. Psychoneuroendocrinology 36(3): 308-29.
35. Sharma S, Fulton S (2013). Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. International Journal of Obesity 37(3): 382-9.
36. Castagné V, Moser P, Roux S, Porsolt RD (2010). Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current Protocols in Pharmacology 49(1): 5-8.
37. Haase J, Brown E (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression-a central role for the
serotonin transporter? Pharmacology &Therapeutics 147: 1-11.
38. Kirilly E, Hunyady L, Bagdy G (2013). Opposing local effects of endocannabinoids on the activity of noradrenergic neurons and release of noradrenaline: relevance for their role in depression and in the actions of CB 1 receptor antagonists. Journal of Neural Transmission 120: 177-86.
39. Ferreira MF, Castanheira L, Sebastiao AM, Telles- Correia D (2018). Depression assessment in clinical trials and pre-clinical tests: a critical review. Current Topics in Medicinal Chemistry 18(19): 1677-703.
40. Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015). The forced swim test as a model of depressive-like behavior. Journal of Visualized Experiments: JoVE. 2(97):52587.
41. Zmudzka E, Salaciak K, Sapa J, Pytka K (2018). Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sciences 210: 106- 24.
42. Lopresti AL, Maker GL, Hood SD, Drummond PD (2014). A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Progress in Neuro-Psychopharmacology and Biological Psychiatry 48: 102-11.
43. Barbosa ML, de Meneses AA, de Aguiar RP, e Sousa JM, Cavalcante AA, Maluf SW (2020). Oxidative stress, antioxidant defense and depressive disorders: a systematic review of biochemical and molecular markers. Neurology, Psychiatry and Brain Research 36: 65-72.
44. Agostinho P, A Cunha R, Oliveira C (2010). Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Current
Pharmaceutical Design 16(25): 2766-78.
45. Naoi M, Maruyama W, Shamoto-Nagai M (2018). Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. Journal of Neural Transmission 125: 53-66.
46. Ren F, Guo R (2021). Synaptic microenvironment in depressive disorder: insights from synaptic plasticity. Neuropsychiatric Disease and Treatment 17:157-165. doi: 10.2147/NDT.S268012. eCollection 2021.
47. Lee KH, Cha M, Lee BH (2020). Neuroprotective effect of antioxidants in the brain. International Journal of Molecular Sciences 21(19):7152.
48. Juan AG, Dargantes AP, Serafica KE, Calonia KM, Awiten RM (2021). Phytochemical and Oral Toxicity Studies of Chromolaena odorata L.(King and Robinson) Leaf Extract. CMU Journal of Science 25(2): 19-25.
49. Fagbohun BO, Unuata ON, Enogela AB, Adewuyi HA, Jonathan I, Gyang DD, Adio WS, Oliwatoyin AH, Mohamed UA, Ogar OE (2024). Phytochemical Characterization, Toxicological Evaluation, and Biological Activities of Phenolics Fractions from Chromoleana Odorata: A Subacute Toxicity Study. Research Square https://doi.org/10.21203/rs.3.rs-5128276/v1
50. Paulose P, Juliet S, Sujith S, Sini M, Divya TM, Nair SN, Chandrasekhar L, Pradeep M, George AJ, Ravindran R (2016). Evaluation of toxicological potential of methanolic extract of Chromolaena odorata found in the Western Ghats of Indian subcontinent orally in mice. Advances in Animal and Veterinary Sciences 4(2): 78-84.
51. Dassamiour S, Bensaad MS, Ghebache W (2024). Utility of phenolic acids in neurological disorders. In Advancement of Phenolic Acids in Drug Discovery 295-344. Academic Press.
52. Sharifi-Rad J, Rapposelli S, Sestito S, Herrera-Bravo J, Arancibia-Diaz A, Salazar LA, Yeskaliyeva B, Beyatli A, Leyva-Gómez G, González-Contreras C, Gürer ES (2022). Multi-target mechanisms of phytochemicals in Alzheimer's disease: Effects on oxidative stress, neuroinflammation and protein aggregation. Journal of Personalized Medicine 12(9):1515.
53. Bellavite P (2023). Neuroprotective potentials of flavonoids: Experimental studies and mechanisms of action. Antioxidants 12(2):280.
54. Ayaz M, Sadiq A, Junaid M, Ullah F, Ovais M, Ullah I, Ahmed J, Shahid M (2019). Flavonoids as prospective neuroprotectants and their
therapeutic propensity in aging associated neurological disorders. Frontiers in Aging Neuroscience 11:155doi: 10.3389/fnagi.2019.00155. eCollection 2019.
55. Ali A, Martins AM, Alam W, Khan H (2023). Neuroprotective effects of alkaloids. In Phytonutrients and Neurological Disorders 245-257. Academic Press.
56. Liew SY, Mak WQ, Thew HY, Khaw KY, Hazni H, Litaudon M, Awang K (2023). Neuroprotective Activities of New Monoterpenoid Indole Alkaloid from Nauclea officinalis. Processes 11(3):646.
57. Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S (2013). Neurosteroids, stress and depression: potential therapeutic opportunities. Neuroscience & Biobehavioral Reviews 37(1): 109-22.
58. Murtala AA, Oyinloye EO, Alabi AO, Aderinola AA, Ogunjimi LO, Odesina OA, Oladoja FA, Shonde OO, Osipitan LE. Datura stramonium abrogates depression-and anxiety-like disorders in mice: possible involvement of monoaminergic pathways in its antidepressant activity. Drug Metabolism and Personalized Therapy 37(3): 305-14.