Chronic consumption of calabash chalk diet induces depression, cognitive impairment and hepatotoxicity in mice
Main Article Content
Abstract
Background: Calabash chalk (CC) is a geophagic material consumed in many parts of the world for pleasure, as a remedy for hyperemesis gravidarum, sialorrhea, and for foetal bone development. Several potential harmful elements are reported to be present in CC.
Objectives: This study evaluated the effect of CC diet on neurobehavioural indices, brain and liver histomorphological changes in mice.
Methods: One hundred and twelve mice were randomly distributed into four major groups of twenty-eight mice per group. Each group was subdivided into four groups of seven mice each and fed with either standard animal feed (control), 10%, 20% or 40% CC diet (treatment groups) for thirty days. Weights of mice were monitored at interval of ten days. On the 31st day, mice from the control and various treatment groups were subjected to either the forced swim test, tail suspension test, three-chamber approach or novel object recognition tests. Twenty-four hours post neurobehavioural tests, animals were sacrificed, their brains and livers isolated, preserved in 10% buffered formalin and used for histological assays.
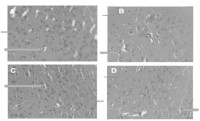
Results: There was a significant increase (p<0.05) in the duration of immobility in the forced swim and tail suspension tests in mice fed with higher concentrations of CC diet. A decrease in contact time spent on the novel object and with the unfamiliar mouse was observed in mice fed with 20 and 40% CC diet. While no cytotoxicity of neuronal and glial cells was observed in this study, hepatotoxicity was noticed in the livers of CC fed mice.
Conclusion: In this study, chronic consumption CC diet produced features characteristic of depression, cognitive dysfunction and hepatic damage in mice.
Downloads
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
Share
References
Abrahams PW, Parsons JA (1996). Geophagy in the Tropics: A Literature Review. The Geographical Journal 162:(1)63-72.
Abraham PW, Follansbee MA, Hunt A, Smith B, Wragg J (2006). Iron nutrition and possible lead toxicity. An Appraisal of geophagy undertaken by pregnant women of UK, Asian communities. Applied Geochemistry 21:98-108.
Abrahams PW, Davies TC, Solomon AO, Trow AJ, Wragg J (2013). Human geophagia, calabash chalk and undongo: mineral element nutritional implications. PLoS One. 8(1):e53304. doi:10.1371/ journal. pone. 0053304.
Reilly C, Henry J (2001). Geophagia: why do humans consume soil? Nutrition Bullet in doi.org/10.1046/j.1467-3010.2000. 00032.x.
Odangowei and Okiemute (2015). Geophagic Practice and Its Possible Health Implications Journal of Sciences and Multidisciplinary Research 7:100-110.
Ekong MB, Ekanem TB, Abraham KE, Akpanabiatu MI, Peter AI, Edagha IA (2012a). Effects of calabash chalk on hematology indices and histomorphology of the spleen of growing Wistar rats. Instasci Journal of Medical Sciences and Clinical Research. 2(1):1-7.
Dean JR, Deary ME, Gbefa BK, Scott WC (2004). Characterization and analysis of persistent organic pollutant and major, minor and trace element in calabash chalk. Chemosphere 57:21 5.
Owhorji B, Okon U, Nwankwo A, Osim E (2019). Chronic consumption of calabash chalk diet impairs locomotor activities and social behaviour in Swiss white Cd-1 mice. Heliyon 5: e01848.
Izugbara, CO. (2003). The cultural context of geophagy among pregnant and lactating Ngwa women of Southeastern Nigeria. African
Anthropology 10(2):180-199.
Vofo BN, Vofo GVFN, Fonge B, Nsagha SD, Egbe TO, Nguedia JC (2019). High umbilical cord blood lead levels and "calabar chalk" consumption amongst pregnant women in two hospitals in Cameroon. The Pan African Medical Journal 109: doi10.11604/pamj.2019.33. 109.13999.
Ekong MB, John EE, Mbadugha CC, Bassey EI, Ekanem TB (2012b). Effect of calabash chalk on the histomorphology of the gastro-oesophageal tract of growing Wistar rats. Malaysian Journal of Medical Sciences 19(1):30-35.
Aprioku JS, Ogwo-Ude EM (2018). Gestational Toxicity of Calabash Chalk (Nzu) in Wistar Rats. International Journal of Applied and Basic Medical Research 4: 249 - 252. doi: 10. 4103/ijabmr.IJABMR_412_17.
Peace GK, Ufuoma EB, Jervas E (2018). Histological studies on the effect of calabash chalk on the cerebellum of gestating Wistar rats. MOJ Anatomy and Physiology 5:(1)50-55.
Ekong MB, Ekanem TB, Sunday AO, Aquaisua AN, Akpanabiatu MI (2012c). Evaluation of calabash chalk effect on femur bone morphometry and mineralization in young wistar rats. A pilot study. International Journal of Applied and Basic Medical Research 2:107-110. doi: 10.4103/2229-516X.106352.
Ekanem TB, Ekong MB, Eluwa MA, Igiri AO, Osim EE (2015). Maternal Geophagy of Calabash Chalk on Foetal Cerebral Cortex Histomorphology. Malaysian Journal of Medical Sciences 22(4)17-22.
Owhorji BI, Okon UE, Osim EE (2018). Calabash chalk chronic diet consumption elevates anxiety and pain perception. World Journal of Pharmaceutical Research 7(12):82-94.
Opara JK Nwagbaraocha EC (2018). The effect of calabash chalk on the uterus of adult female Wistar rats. GSC Biological and Pharmaceutical Sciences 5(2):26-31.
Ekong MB, Peter AI, Ekanem TB, Eluwa MA, Mbaduga CC, Osim EE (2014). Calabash chalk's geophagy affects gestating rats' behaviour and the histomorphology of the cerebral cortex. International Journal of Brain Science https://doi.org/10.1155/2014/394847.
Sanders T, Liu Y, Buchner V. Tchounwou PB (2009). Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Reviews on Environmental Health 24(1):15-45.
Berk M, Williams LJ, Andreazza AC, Pasco JA, Dodd S, Jacka, FN et al (2014). Pop, heavy metal and the blues: Secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. British Medical Journal,4(7):e005142-e005142.
Orisakwe OE (2014). The Role of Lead and Cadmium in Psychiatry. North American Journal of Medical Sciences. 6(8):370-376.
Ayuso-Álvarez A, Simón O, Nuñez C, RodríguezBlázquez I, Martín-Méndez A, Bel-lán G et al (2019).Association between heavy metals and metalloids in topsoil and mental health in the adult population of Spain. Environmental Research https://doi.org/10.1016/ j.envres.2019.108784.
Lahouaoui H, Benammi H, Aimrane A, El Hidan AM, Khamsi Y, Draoui A (2019). Depression and Anxiety Emerging from Heavy Metals: What Relationship? Available at 10.4018/978-1-5225-7775-1.ch015. Accessed 15th January, 2021.
Wang X, Huang X, Zhou L, Chen J, Zhang X, Xu K et al (2021). Association of arsenic exposure and cognitive impairment: A population-based crosssectional study in China. NeuroToxicology 82:100-107.
Stanley PC, Wakwe VC (2002). Toxic trace metals in the mentally ill patients. Nigerian Postgraduate Medical Journal 9:199-204.
Shiue I. (2015). Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011-2012. Environmental Science and Pollution Research 22(21):17095-17103.
Zaw YH, Taneepanichskul N (2019). Blood heavy metals and brain-derived neurotrophic factor in the first trimester of pregnancy among migrant workers. PLoS ONE 14(6):1-13.
NIH, Public Health Service Policy on Humane Care and Use of Laboratory Animals (2015). http://grants.nih.gov/grants/olaw/references/PHSPolicy LabAnimals.pdf.
Steru L, Chermat R, Thierry B and Simon P (1985). Tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367-370.
Porsolt, R.D., Anton, G., Blavet, N. and Jalfre, M. (1978) Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. European Journal of Pharmacology 47:379-391.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR et al (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behaviour in mice. Genes, Brain and Behaviour 3:287-302.
Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C et al (2009). Abnormalities in brain structure and behaviour in GSK-3alpha mutant mice. Molecular Brain 2: 35 https://doi.org/10.1186/1756-6606-2-35.
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR (2011). Assessment of social interaction behaviours. Journal of Visualized Experiments (48):2473. doi:10.3791 /2473.
SBFNL (Stanford Behavioural and Functional Neuroscience Laboratory) 2019, Available at https://med.stanford.edu/sbfnl/services/bm/si/three-chamber.html. Accessed 19th June, 2019.
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioural data. Behavioural Brain Research 1:31(1):47-59. doi: 10.1016/0166-4328(88)90157.
Antunes M, Biala G (2012). The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing 13(2):93- 110. doi: 10.1007/s10339-011-0430-z.
Slaoui M, Fiette L (2011). Histopathology Procedures: From Tissue Sampling to Histopathological Evaluation Jean-Charles Gautier (ed.), Drug Safety Evaluation: Methods and Protocols, Methods in Molecular Biology, vol. 691, DOI 10.1007/978-1-60761-849-2_4, Springer Science Business Media, LLC.
de Souza IBMB, Costa LFR, Tiago PRF, Cagni FC, Lima RH, Silva ED Jr et al. (2019). Venlafaxine and nortriptyline reverse acute dexamethasone-induced depressive-like behaviours in male and female mice. Experimental and Clinical Psychopharmacology 27(5):433-442.
Porsolt RD, Chermat R, Lenègre A, Avril I, Janvier S, Stéru L.Arch Int (1987). Use of the automated tail suspension test for the primary screening of psychotropic agents. Archives Internationales de Pharmacodynamie et de Therapie 288(1):11-30.
Cryan JF, Markou A, Lucki I (2002). Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences 23:238-245. doi: 10.1016/S0165-6147(02)02017-5.
Crawley JN (2004). Designing mouse behavioural tasks relevant to autistic-like behaviours. Mental Retardation and Developmental Disabilities Research Reviews 10 (4): 248-258. doi: 10.1002/mrdd.20039.
Suha AJ, Hosseinmardi N, Janahmadi M (2022). Spatial working memory is disparately interrelated with social status through different developmental stages in rats. Behavioural Brain Research 416:113547 doi.org/10.1016/j.bbr.2021.113547.
Taborsky B, F.Oliveira F (2012) Social competence: an evolutionary approach Trends in Ecology and Evolution 27(12):679-688.
Porwal M, Khan NA, Maheshwari KK (2017). Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia
tenacissima Leaves in Experimental Rats. Scientia pharmaceutica 85 (3): 29. doi:10.3390/scipharm85030029.
Bellinger DC (2008). Very low lead exposures and children's neurodevelopment. Current Opinion in Pediatrics 20(2):172-177.
Chang CY, Schiano TD (2007). Review article: drug hepatotoxicity. Aliment Pharmacology and Therapeutics. 25(10):1135-1151.
Lackner C, Gogg-Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H (2008). Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. Journal of Hepatology 48 (5): 821 - 828. doi: 10.1016/j.jhep.2008.01.026.
Das BK, Choukimath SM, Gadad PC (2019). Asarone and metformin delays experimentally induced hepatocellular carcinoma in diabetic milieu. Life Sciences 230(1):10-18.
Ekong MB, Akpantah AO, Ibok OS, Eluwa MA, Ekanem TB (2009). Differential effects of calabash chalk on the histology of liver of adult Wistar rats Internet J Health. 8 (2). Available at; http://www.ispub.com/journal/the_internet_journal_of_health/volume_8_number_2_12/article/differentia_effects_of_calabash_chalk_on_the_histology_of_the_liver_of_adult_wister_rats.html. Accessed October 11th, 2019.
Ekong MB, Aniekan PI, Akpanabiatu MI, Ekanem TB, Eluwa MA (2013). Potency of calabash chalk on the liver function and histomorphology. Journal of Medical Research and Practice 2(7):203-207.
Panqueva L, Pilar, R. (2013). Hepatopathology for gastroenterologists and hepatologists. Part Two: Useful terminology in the interpretation of the histopathological findings. Revista colombiana de Gastroenterología 28(2):152-159.
Bianchi FB, Biagini G, Ballardini G, Cenacchi G, Faccani A, Pisi E et al., (1984). Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 4(6):1167-1172.
Walsh KM, Fletcher A, MacSween RN, Morris AJ (2000). Basement membrane peptides as markers of liver disease in chronic hepatitis C. Journal of Hepatology 32:325-330.